Thermodynamics and Phase Diagram Prediction of Hydrides
Category
Modeling/Simulation
Laboratory
Lawrence Livermore National Laboratory (LLNL)
Capability Experts
Brandon Wood ([email protected]), Tae Wook Heo ([email protected]), ShinYoung Kang ([email protected]), Stanimir Bonev ([email protected]), Tadashi Ogitsu ([email protected])
Description
Because hydrogen storage materials are cycled under varying H2 pressures and temperatures, understanding stability of various hydride phases at given conditions is crucial for promoting desired reaction pathways while suppressing formation of unwanted products. In addition, the thermodynamics of metal hydrides can be affected by particle size or by mechanical stresses exerted by external confining media. HyMARC provides access to a diverse toolkit for accurate prediction of thermodynamics using high-performance computing. These calculations can be used to construct phase diagrams for hydrogen uptake and release, providing guidance for optimizing pressure- and temperature-dependent thermodynamic pathways.
In particular, our capabilities include:
- Finite-temperature free energy calculations
- Size- and environment-dependent stability of phases
- Effects of internal and confinement stresses on reaction enthalpies.
Finite-Temperature Free Energy Calculations
The phase stability of metal hydrides relies on accurate computation of both reaction enthalpy and entropy. We apply density functional theory, validated and calibrated via experimental input, to determine reaction enthalpies. To understand the effects of temperature, we apply ab initio molecular dynamics to capture anharmonic features in the entropy of complex metal hydrides that are neglected in conventional simulations. This method can also resolve entropic contributions from specific atoms, regions of space, or normal modes.
Size- and Environment-Dependent Stability
Insight into how reaction enthalpies can change upon nanosizing can be obtained by mixing bulk and surface energies. In addition, these energies can be modified to account for different chemical environments that can be present during (de)hydrogenation.
Effects of Mechanical Strain and Confining Medium
Cycling of hydrogen storage materials can create internal stresses due to lattice mismatch, as can the presence of external confining media. We combine first-principles calculations, molecular dynamics, and continuum modeling to allow for the determination of mechanical strain contributions to the reaction enthalpy based on elastic properties.
Figures
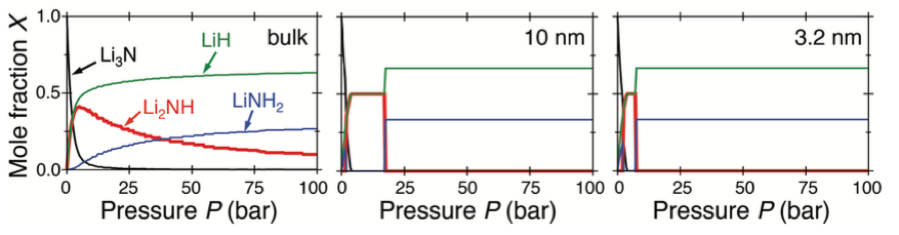
Predicted equilibrium mole fractions X of Li3N (black), Li2NH (red), LiNH2 (blue), and LiH (green) as a function of H2 pressure P upon isothermal hydrogenation of the bulk material (left) and particles of diameter d = 10 nm (center) and d = 3.2 nm (right) [1].
References
- B. C. Wood, V. Stavila, N. Poonyayant, T. W. Heo, K. G. Ray, L. E. Klebanoff, T. J. Udovic, J. R. I. Lee, N. Angboonpong, J. D. Sugar and P. Pakawatpanurut, “Nanointerface-driven reversible hydrogen storage in the nanoconfined Li-N-H system,” Adv. Mater. Interfaces 4 (2017): 1600803.
- X. W. Zhou, T. W. Heo, B. C. Wood, V. Stavila, S. Kang, and M. D. Allendorf, “Finite-temperature PdHx elastic constants computed by direct molecular dynamics,” MRS Adv. 2 (2017): 3341.
