Synthesis of Metal Hydride Composites
Category
Synthesis
Description
A scalable bottom-up synthetic route to create atomically defined and tunable graphene-based materials as stabilizing support for metal hydride and complex hydride nanoparticles has been developed. The light weight carbon-based matrix serves as a semipermeable support, mediates the thermal energy exchange during absorption/desorption, prevents undesired oxidation and sintering of nanoparticles, and is inert to pressurized hydrogen at elevated temperatures. Furthermore, the matrix is transparent to TEM and X-ray spectroscopy enabling sample preparation and in situ investigation of phase transitions within isolated metal hydride and complex hydride nanoparticles.
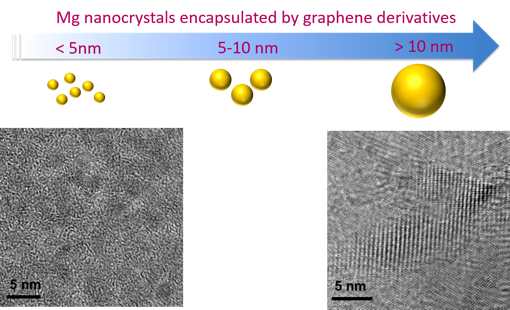
This approach was employed to Mg and MgH2 nanocrystals, which were synthesized using a solution-based approach that is easily scaleable.1,2 Here, graphene oxide (GO) sheets, which are selectively permeable to hydrogen, were employed as an encapsulation layer. The resulting GO-encapsulated Mg crystals exhibit excellent air stability and hydrogen storage capacity.3 This synthetic approach is now being expanded beyond the initial concept to other graphene derivatives to examine the effect of different kinds of graphene layers on hydrogen storage performance and the stability of Mg crystals (Figure 1). Future work will focus on forming composites using complex metal hydrides, including boron hydrides.
Status
Available for Mg and MgH2 only, in collaboration with HyMARC; extension to other hydrides is in progress.
Figures
References
- K. J. Jeon, et al., "Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts," Nat Mater 10 (2011): 286–290.
- A. M. Ruminski, R. Bardhan, A. Brand, S. Aloni, J. J. Urban, "Synergistic enhancement of hydrogen storage and air stability via Mg nanocrystal-polymer interfacial interactions," Energ Environ Sci 6 (2013): 3267–3271.
- E. S. Cho, et al., "Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage," Nat Commun 7 (2016): 10804.
