Room Temperature Hydrogen Storage Framework Materials
Category
Synthesis
Laboratory
Lawrence Berkeley National Laboratory (LBNL)
Description
Metal–organic framework materials containing coordinatively unsaturated metal sites exhibit large hydrogen usable capacities at near ambient temperature compared to conventional porous materials and metal hydrides. The HyMARC team demonstrated that one of the top-performing adsorbent materials, Ni2(m-dobdc) (m-dobdc4– = 4,6-dioxido-1,3-benzenedicarboxylate), exhibits the highest physisorption H2 storage capacity to date for an adsorbent operating at 298 K and pressures up to 100 bar. Notably, when used in a process with temperature swings between –75 and 25 °C, this material achieves a usable volumetric capacity of 23.0 g/L in the pressure range of 5–100 bar. This is attributable to the high adsorption enthalpy of the adsorbents and the high volumetric density of such metal sites per volume.
Status
Online and available for use in collaboration with HyMARC.
Figures
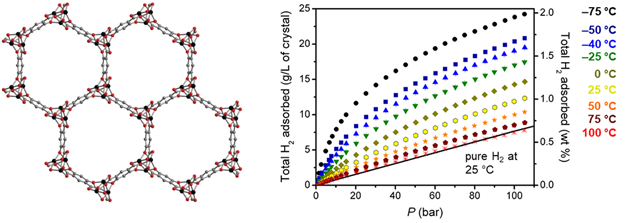
Figure 1. (Left) Crystal structure of M2(m-dobdc) (M = Ni, Co, Mn, Fe, Mg) showing one-dimensional hexagonal pores and helical metal chains. (Right) Total H2 isotherms for Ni2(m-dobdc) plotted in terms of total volumetric and gravimetric capacity. The black line represents the volumetric density of pure compressed H2 at 25 °C.
References
- 1. M. T. Kapelewski, T. Runcevski, J. D. Tarver, H. Z. H. Jiang, K. E. Hurst, A. Ayala, T. Gennett, S. A. FitzGerald, C. M. Brown and J. R. Long, Chem. Mater. 30 (2018): 8179.
